SHARE WITH FRIENDS:
Adults. Physical quantities
Plan:
-
. Sizes and their types.
-
. Units of magnitude.
-
. Size of adults.
-
. Rules for defining and writing units and dimensions.
-
. International system of units.
Basic phrases: magnitude, unit of magnitude, basic unit, derived unit, size of magnitude, value of magnitude, International System of Units.
3.1. Sizes and their types
Size – It is a property that is qualitatively general to many physical objects (physical systems, their states and processes occurring in them), and quantitatively is a property that is specific to each object.
A particularity in the definition means that the property of one object is greater or lesser than that of another.
The science of metrology is closely related to these quantities, their units, and the development of measurement techniques.
Each physical object can be characterized by a number of objective properties. With the progress and development of science, the demand for knowledge of these properties is increasing. By now, it is possible to measure more than 70 quantities with the help of modern measuring tools. This figure is predicted to exceed 2050 by 200.
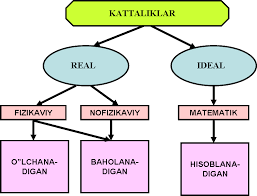
The division of quantities into types is shown in figure 3.1.
3.1. - a picture. Types of adults.
3.2. Size of size
Since each property can be expressed to a greater or lesser degree, that is, have a quantitative description, it means that this property can also be measured.
We use dimensionality to express qualitative descriptions of quantities in a formal way.
As a measure of size, which indicates the relationship of this quantity with the main quantities in the system and the proportionality coefficient is equal to 1.
Size of sizes dimension - size, size based on the meaning of the (visual) word dim is denoted by the symbol
Usually, the size of the main quantities is indicated by appropriate capital letters, for example: length - diml = L; mass - dim m = M; time dim t = T.
When determining the size of derivative quantities, the following rules should be followed:
-
The dimensions of the right and left sides of the equation cannot match, because only identical properties can be compared. To conclude from this, we can algebraically add only quantities that have the same dimension.
-
Algebra of dimensions is multiplicative, that is, it consists only of multiplication.
2.1. The size of the product of several quantities is equal to the product of their sizes, that is: if the connection between the values of the quantities A,V,S,Q is given in the form Q=AVS, then:
dim Q = (dim А) (dim V)(dimС)
2.2. The size of the division when dividing one quantity by another is equal to the ratio of their sizes, that is, if Q = A/V, then:
dim Q = dim А/ dim V
2.3. The dimension of an arbitrary quantity raised to a power is equal to its dimension increased by that degree, i.e. Q = Ap if In that case:
dim Q = dim pА
for example, if the velocity is v=l/t. In that case:
dim v = diml/ dim t = L/T= LT - 1
thus, we can use the following formula to express the size of the derivative quantity:
dim Q = LpMmTk….,
where, L, M, T.., are the size of the main quantities, respectively;
n,m,k.., is the scale index.
The exponent of each dimension can be positive or negative, integer or fractional, or zero. If all degree exponents are equal to zero, then such a quantity is called a dimensionless quantity. This quantity can be relative (for example, dielectric conductivity), logarithmic (for example, the logarithmic ratio of electric power and voltage), determined by the ratio of quantities of the same name.
Dimensional theory is usually very useful for checking generated expressions (formulas). Sometimes this check allows to find unknown quantities.
3.3. Units of magnitude
Since the quantity describing a particular object has a quantity description specific to that object, such objects, when viewed together, differ only according to these quantity descriptions. For this, there must be some basis between objects when comparing. This basis is called the unit of comparison. It is the basis of such a description that is called the unit of magnitude.
Its size serves as a quantitative description of an arbitrary property of the considered physical object. The concepts of size and value should not be confused with each other. For example, 100 g, 105 mg, 10 - 4 t is the expression of one size in 3 different forms, usually we say "mass (...) kg" instead of "value of mass size". So, by the value of a quantity, we should understand that its size is expressed in a certain number of units.
Size of size - is the quantity of the quantity that belongs to some taken material object, system, phenomenon or process.
The value of size - to determine the quantitative description of a quantity with a certain number of accepted units.
The component of the value expressed in numbers is called the numerical value of the quantity. The numerical value indicates how many units the size of the quantity differs from zero, or how many units are larger (smaller) than the size taken as a unit of measurement, or in other words, the value of the quantity Q is the size of its unit of measurement [Q] and its numerical value q we must understand the meaning expressed by:
Q = q[Q].
The unit of magnitude is also divided into basic and derived units similar to the magnitude itself (Figures 3.2 and 3.3).
3.2 - picture. Types of units of magnitude.
3.3 - picture. Units of magnitude.
3.4. Units and o 'sizing and writing rules
There are standards-based procedures and rules for defining and writing units of magnitude. These rules and procedures are based on UzDSt 8.012:2004 "State system of ensuring the unity of measurements. It is described in detail in the standard "Units of Magnitudes".
-
Special letters or symbols can be used to represent units – A, W, %, etc.
-
The letter representing the unit is written in the correct font. Use of period for abbreviation is not allowed.
-
The unit sign is expressed after the numerical value of the quantity, along with it, without passing the next one. The character with the last digit of the number value is written in a space of one space.
Correct: Incorrect:
100 kW 100 kW
80% 80%
20 0S 200S or 200 С
(Excluding superscripts)
250 20 0
-
When expressing a numerical value with a decimal point:
Correct: Wrong
423,06 m 423 m, 06
5,7580 or 5045,48 50, 758 or 5045, 48
5045.28,8 5045.28..,8
-
When a value range is displayed
Correct: False
(100,0±0,1) kg 100,0±0,1 kg
50 mm ± 1 mm 50 ± 1 mm
-
In the graphs of the tables and at the beginning of the rows, you can generally give a unit sign.
-
In cases expressed by the formula, to give in the form of an explanation:
Correct: False
v = 3,6 s/t v = 3,6 s/t km/h
where: v – speed, km/s where: s – distance, m,
s – distance, m t – time, s
t – time, s
-
A dot can be placed at the middle height of the letter when characters are displayed as multiples
Tbulletre: Notbulletri
Nm Nm
Pa.s Pas
-
You cannot use more than one decimal point in a fractional expression.
-
When expressing unity:
Correct: False
W/ (ms) W/ms
80 km/h 80 km/s - t
80 kilometers per hour 80 kilometers per hour
3.5. International system of units
In 1960, the X1 General Conference of Weights and Measures adopted the international system of units, which in our country is called the SI (SI - Systeme International) system of international units.
In subsequent general conferences, a number of changes were made to the SI system.
The SI system has been introduced in our country since 1982 and has its own advantages (Figure 3.4). In 2004, Uz DSt 8.012:2004 (State system of ensuring the unit of measurements of the Republic of Uzbekistan. Units of sizes) was adopted.
The basic units of the international system of units (Uz DSt 8.012:2004) are given in table 1.
The first three basic units - meter, kilogram, second (table 3.1) are derivative units that coordinate all units with mechanical properties, and the rest, that is, the units of electric current, thermodynamic temperature, quantity of matter and light intensity, are in accordance with mechanical units. corrects the possibility of forming non-derivative units: ampere - electrical and magnetic quantities, kelvin - thermodynamic temperature, candelas – light and mol - in the fields of molecular physics and chemistry.
3.4 - picture. Advantages of the international system of units.
Table 3.1
The main units of the International System of Units (SI).
Bigness |
Birlik |
||||
Nomi |
O 'call—leagues |
Nomi |
Symbol |
Ta'rife |
|
SI tall— cha |
Ruscha |
||||
1 |
2 |
3 |
4 |
5 |
6 |
Length |
L |
meter |
m |
m |
A meter is the distance light travels in a time interval of 1/299792458 s. |
Sponge |
M |
Kilo - gram |
kg |
kg |
The kilogram is a unit of mass equal to the mass of the international kilogram prototype |
Time |
T |
second |
s |
с |
A second is 133 periods of radiation corresponding to the transition between two ultrafine levels of the ground state of a cesium-9192631770 atom. |
Electric current (power of electric current |
I |
ampere |
A |
А |
An ampere is a current passing through two parallel straight conductors of infinitely long, very small circular diametrical cross-section, located 1 m apart in a vacuum, at a rate of 1·2 per 10 m length of the conductor. - 7 A constant current force capable of producing an interaction force equal to N. |
Thermo-dynamic temperature |
Θ |
kelvin |
K |
K |
Kelvin is a unit of thermodynamic temperature equal to 1/273,16 of the thermodynamic temperature of the triple point of water. |
Amount of substance |
N |
mol |
Mol |
Mol |
A mole is the amount of a system that contains as many elements as carbon-0,012 with a mass of 12 kg. In the application of mole, the elements must be grouped and they can consist of groups of atoms, ions, electrons and other particles. |
The power of light |
J |
candelas |
Cd |
Kd |
A candela is 540·10 in a given direction12 It is the light power of a source that emits monochromatic radiation with a frequency of Hz and the energy light power in this direction is 1/683 W|sr. |
Derived units of the International System of Units (SI).
Derived units of SI are derived according to the rules for forming coherent derived units of SI. Examples of derived SI units derived using SI base units are given in Table 3.2.
Table 3.2
Names and symbols consist of the names and symbols of the main units
found Examples of SI derived units
Bigness |
Birlik |
||
Nomi |
Size |
Nomi |
Symbol |
Square |
L2 |
square meter |
m2 |
volume, capacity |
L3 |
cubic meter |
m 3 |
Speed |
LT - 1 |
meters per second |
m / s |
Acceleration |
LT - 2 |
meter divided by second squared |
m / s2 |
Density |
L - 3M |
The division of kilograms into the cube of a meter |
kg / m3 |
Wave number |
L - 1 |
the degree of the meter is minus one |
m - 1 |
Comparative size |
L3M - 1 |
cubic meters divided by kilograms |
m3/ kg |
Electric current density |
L - 2I |
ampere distribution meter squared |
A/m2 |
Magnetic field strength |
L - 1I |
ampere distribution meter |
A/m |
The molar concentration of the component |
L - 3N |
mol distribution is the cube of a meter |
mol / m3 |
Clarity |
L - 2J |
candela distribution meter squared |
cd / m2 |
Derivative units of SI with special names and designations are shown in table 3.3.
Table 3.3
SI Derived units with a special name and designation
Bigness |
Birlik |
|||
Nomi |
Size -leagues |
Nomi |
Symbol |
Representation by SI base and derived units |
1 |
2 |
3 |
4 |
5 |
Flat corner |
l |
radians |
rad |
m×m - 1 =1 |
Spatial angle |
l |
steradian |
sr |
m2×m - 2 = l |
Frequency |
T - 1 |
gers |
Hz |
s - 1 |
Kuch |
LMT - 2 |
newton |
N |
m×kg×s - 2 |
Pressure |
L - 1MT - 2 |
pascal |
Pa |
m - 1× kg × s - 2 |
Amount of energy, work, heat |
L2MT - 2 |
joule |
J |
m2× kg × s - 2 |
Power |
L2MT - 3 |
watt |
W |
m2× kg × s - 3 |
1 |
2 |
3 |
4 |
5 |
Electric charge, amount of electricity |
TI |
kulon |
С |
s×A |
Electric voltage, electric potential, electric potential difference, electric driving force |
L2MT - 3I - 1 |
volt |
V |
m2× kg × s - 3 - A - 1 |
Electrical capacity |
L-2M -1T4I2 |
farad |
F |
m - 2× kg - 1s4A2 |
Electrical resistance |
L2M - 1T3I2 |
om |
Ω |
m2× kg × s - 3 A2 |
Electrical conductivity |
L-2M1T -3I-2 |
Siemens |
S |
m - 2× kg - 1×p3 A - 2 |
Current of magnetic induction, magnetic flux |
L2MT -2I -1 |
weber |
Wb |
m2× kg × s - 2×A - 1 |
Magnetic flux density, magnetic induction |
MT -2I -1 |
tesla |
T |
kg×s - 2×A - 1 |
Inductance, mutual inductance |
L2MT - 2I - 2 |
henry |
H |
m2× kg × s - 2×A - 2 |
Celsius temperature |
θ |
Celsius degrees |
0С |
K |
The flow of light |
J |
lumen |
lm |
cd×sr |
Illumination |
L - 2J |
luxury |
Ix |
m - 2×cd×sr |
Activity of nuclides in a radioactive source (activity of a radionuclide) |
T - 1 |
becquerel |
Bq |
s - 1 |
Absorbed dose of ionizing radiation, kerma |
L2T - 2 |
grey |
Gy |
m2s - 2 |
Equivalent dose of ionizing radiation, effective dose of ionizing radiation |
L2T - 2 |
zivert |
Sv |
m2s - 2 |
Catalyst activity |
NT - 1 |
catal |
cat |
goods - s - 1 |
Rules for forming the names and symbols of units of the International System of Units, decimal multiples and fractional units
The names and designations of SI decimal and fractional units are formed using the multipliers and prefixes listed in Table 3.4.
3.Table 4
Multipliers and prefixes used to form the names and designations of SI decimal and fractional units
Decimal Multiplier |
Prefix |
Prefix symbol |
Decimal multiplication—who |
Prefix |
Prefix symbol |
1024 |
iota |
Y |
10 - 1 |
sea |
d |
1021 |
zetta |
Z |
10 - 2 |
Saints |
с |
1018 |
axis |
Е |
10 - 3 |
milli |
m |
1015 |
heel |
R |
10 - 6 |
mikro |
μ |
1012 |
tera |
T |
10 - 9 |
dwarf |
n |
109 |
jig |
G |
10 - 12 |
piko |
p |
106 |
mega |
M |
10 - 15 |
femto |
f |
103 |
kilo |
k |
10 - 18 |
atto |
а |
102 |
hecto |
h |
10 - 21 |
Zepto |
z |
101 |
deka |
da |
10 - 24 |
iocto |
y |
It is not allowed to add two or more consecutive prefixes to the name or symbol of the unit. For example, the unit name should be written picofarad instead of micromicrofarad.
Notes:
-
Since the name of the basic unit - kilogram has the prefix "kilo", the fractional unit of mass - gram (0,001 kg) is used to create multiple and percentage units of mass, and the prefixes are written by adding the word "gram" should, for example, milligram (mg) instead of microkilogram (µkg).
-
It is allowed to use the percentage unit of mass - gram without a prefix (unit symbol - g).